My Store
Electrolytes| Has It Effervescent tablets
Electrolytes| Has It Effervescent tablets
Couldn't load pickup availability
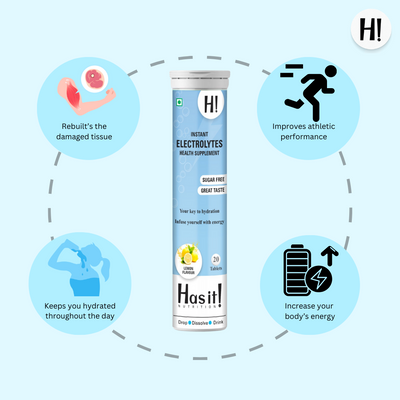
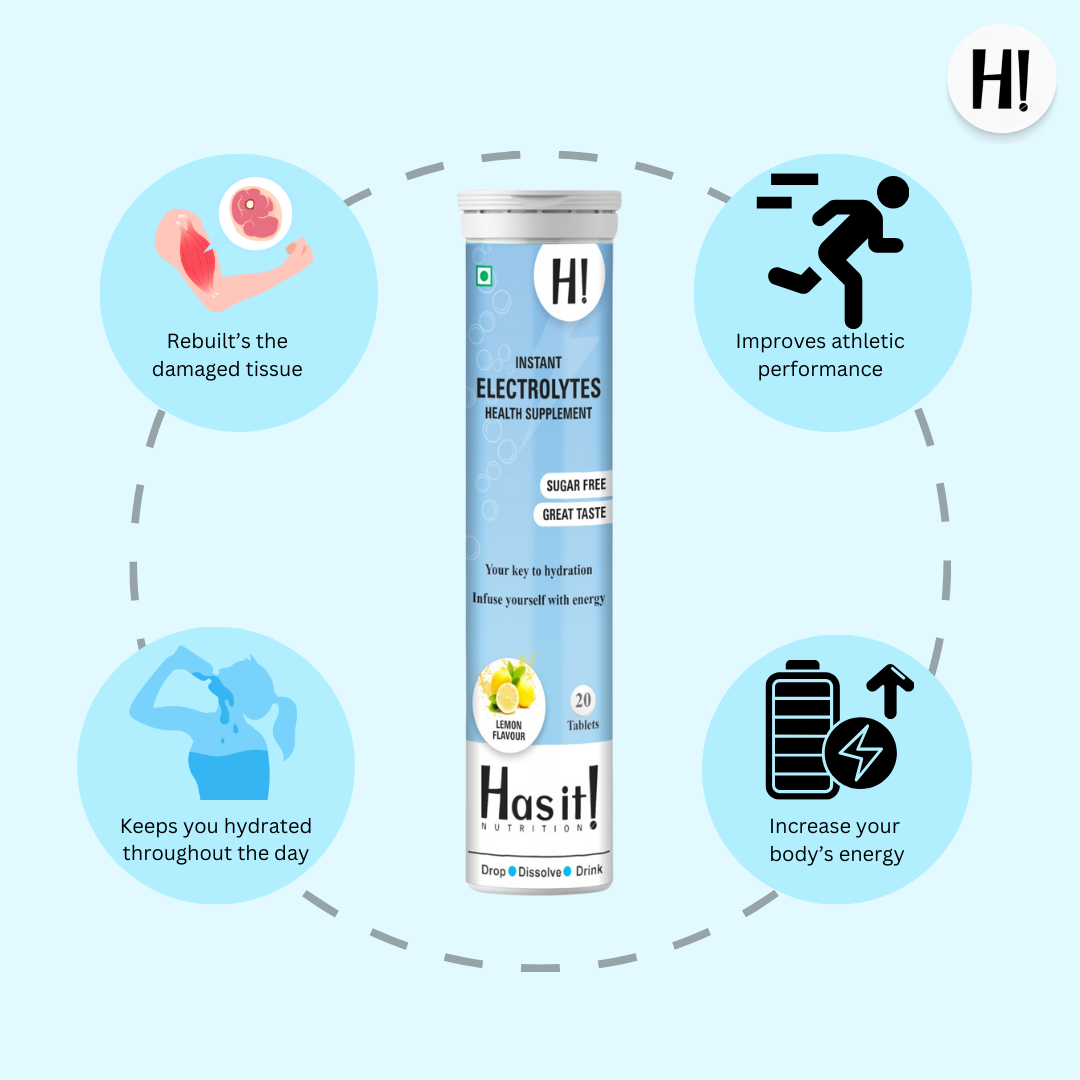
Our instant Electrolytes tablets provide double the energy and hydration. They have the right balance of 5 essential electrolytes, i.e, Sodium, Potassium, Magnesium, Calcium and Chloride.
Each of our instant electrolyte bottles contains 20 effervescent tablets. Compared to other hydration and energy drinks, it has zero sugars.
When to expect an outcome?
Majorly, it takes 4-5 weeks to have a visible result
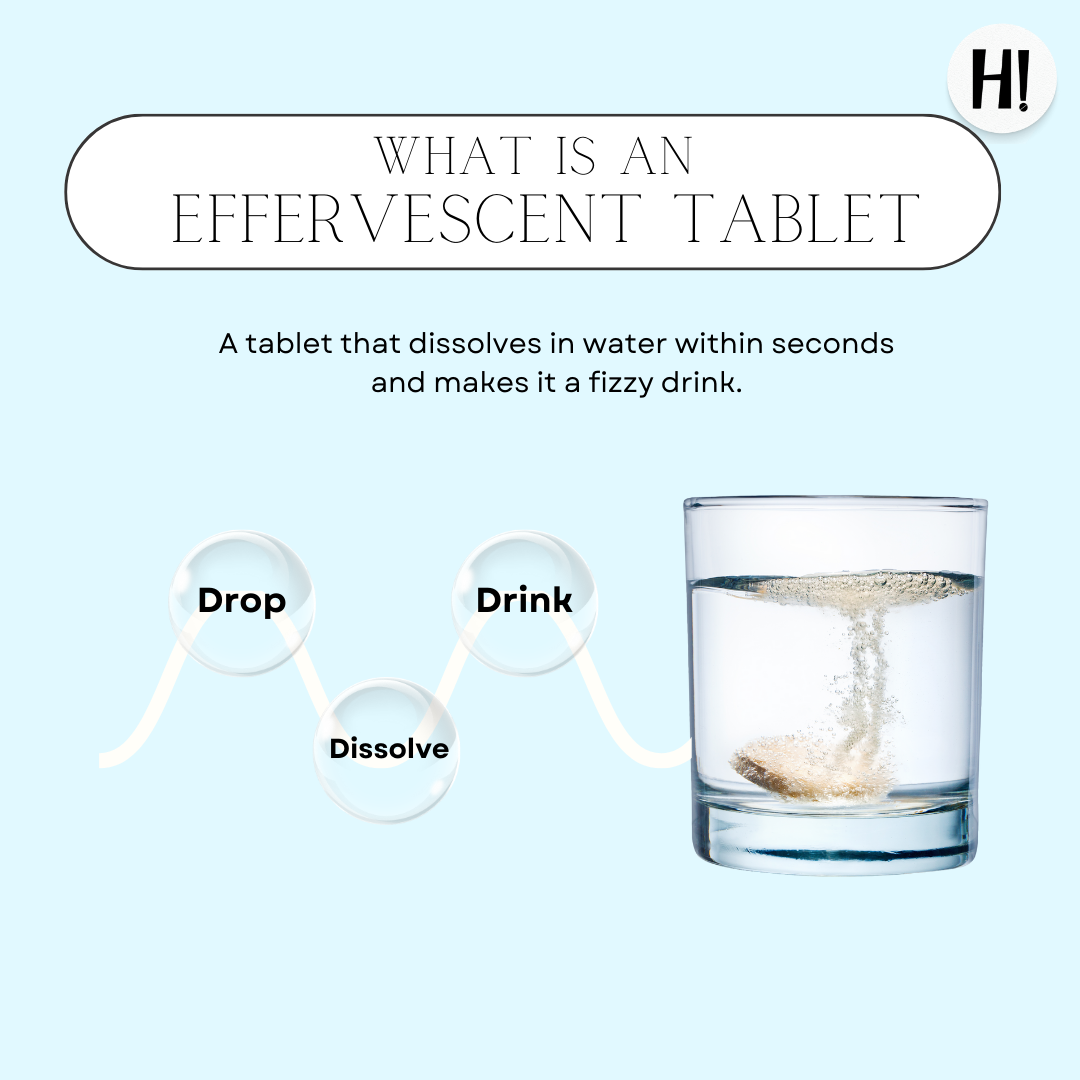
How to consume?
- Easy–Peezy!
- Drop a tablet in 200 ml of water.
- Let it Fizzz.
- Drink the tasty Fizzy Drink.
The Electrolytic Perspective
Remember back in eighth grade when we learned about electrolysis? Well, our body has a process of electrolysis of its own. A few days back, we were working on this formula of Electrolytes when we connected the dots. It must have been long since you have passed the eighth grade. Let’s revise how electrolysis works. In a solution, there are two electrodes. The positive electrode is called cathode, while the negative electrode is called anode. When an electric current passes through the solution, the compound in it breaks into ions. The positive ions, also known as cations are attracted to anode. On the other hand, the negative ions, also known as anions are attracted to cathode. For example, in a salt solution, the salt compound breaks down into the positively charged sodium ions (Na+), which are attracted to the negative electrode (cathode). On the other hand, the negatively charged chloride ions (Cl-) are drawn to the positive electrode (anode).
These ions are parties to electrolysis and are thus also known as electrolytes.
Coming to the cellular level, electrolytes constantly move in and out of our cells through a process called osmosis.
Osmosis is the movement of water molecules across a semipermeable membrane from an area of low solute concentration (e.g., inside the cell) to an area of high solute concentration (e.g., outside the cell). Electrolytes play a critical role in regulating this movement of water, ensuring that our cells maintain the right balance of fluids for optimal function.
Our bodies constantly lose electrolytes through sweat, urine, and even normal bodily processes. To maintain optimal health, it is essential to replenish these essential minerals.

















Amazing product




